On a recent blood test, my plasma level of homocysteine (Hcy) was 11.9 uMol. Is that optimal minimizing disease risk and maximizing longevity? Let’s have a look at the literature.
A 2017 meta-analysis of 11 studies including 27,737 participants showed an increased risk of death from all causes (“all-cause mortality”; ACM) as circulating levels of homocysteine increase (Fan et al. 2017):
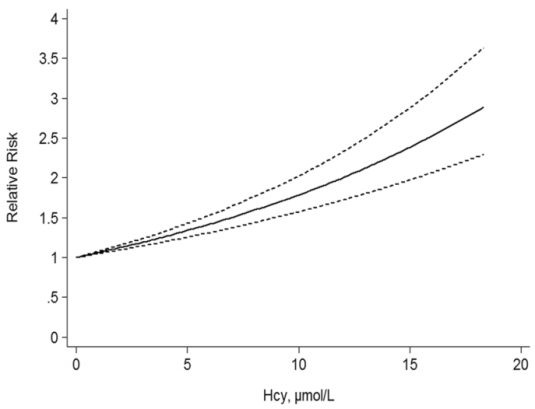
When looking at meta-analyses, it’s important to examine each of the individual studies. Here are the data for the 11 included studies:
- Kark et al. 1999: 1,788 older adults, average age 65y, followed for 9-11 years. Compared with values less than 8.5 uMol, subjects with elevated homocysteine (> 14.7) had a 2-fold higher risk of death from all causes.
- Bostom et al. 1999: 1,933older adults, verage age, 70y, median follow-up, 10y. Subjects with values > 14.3 uMol had 2-fold ACM risk, when compared with < 14.3.
- Hoogeveen et al. 2000: 811 older adults (average age, 65y), 5 yr follow-up. Non- diabetics had a 34% increased ACM risk (p=0.08), but diabetics had 2.5-fold increased ACM risk after a 5-yr follow-up.
- Vollset et al. 2001: 4,766 older adults (age range, 65-67y at study entry), median 4 yr follow-up. Compared with 5.1-8.9 uMol, values greater than 12 were significantly associated with a 2.4-4.5 increased ACM risk.
- Acevedo et al. 2003. 3,427 subjects, average age 56y, ~3yr follow-up. ACM risk lowest for < 9.4 uMol, compared with > 14.4.
- González et al. 2007: 215 older adults (average age, 75y), median 4 yr follow-up. Compared with < 8.7 uMol, values > 16.7 had 2.3-fold increased ACM risk.
- Dangour et al. 2008: 853 older adults (average age, 79y), ~7.6y follow-up. Homocysteins > 19.4 uMol associated with ~2-fold higher ACM risk, when compared with < 9.8.
- Xiu et al. 2012: 1,412 older adults (average age, ~75y), up to 10 year follow-up. 1.8-fold higher ACM risk comparing those with >14.5 uMol with < 9.3.
- Waśkiewicz et al. 2012: 7,165 middle aged adults, ~5yr follow- up. 1.8-fold increased ACM risk for subjects with homocysteine > 10.5 uMol(average age, 52y) when compared with < 8.2 (avg age, 40y).
- Wong et al. 2013: 4,248 older men, average age ~77y, ~5yr follow-up. 1.5-fold increased ACM risk for homocysteine values > 15 uMol.
- Swart et al. 2012: 1,117 older adults (average age, 75y), up to a 7yr follow-up. In 543 men, homocysteine was not associated with ACM risk. In 574 women, 1.7 to 1.9-fold higher ACM risk when comparing > 12.7 and >15.6 vs < 10.3 uMol.
Not included in their analysis:
- Petersen et al. 2016: 670 subjects, average age 65y, average follow-up 14.5y. Subjects with homocysteine values ≥ 10.8 μmol/l had a significant higher incidence of all-cause mortality:

In sum, the evidence appears consistent across these 12 studies that elevated homocysteine is associated with an increased risk of death from all causes. Based on the Fan et al. (2016) meta-analysis, lower appears better, with values < 5 uMol associated with maximally reduced ACM risk. Also based on that data, my ACM risk is ~1.5-fold increased!
To see how dietary changes and supplements have impacted my homocysteine levels, see this link: https://michaellustgarten.wordpress.com/2018/03/23/reducing-homocysteine-updates/
If you’re interested, please have a look at my book:
References:
Bostom AG, Silbershatz H, Rosenberg IH, Selhub J, D’Agostino RB, Wolf PA, Jacques PF, Wilson PW. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med. 1999 May 24;159(10):1077-80.
Dangour AD, Breeze E, Clarke R, Shetty PS, Uauy R, Fletcher AE. Plasma homocysteine, but not folate or vitamin B-12, predicts mortalityin older people in the United Kingdom. J Nutr. 2008 Jun;138(6):1121-8.
Fan R, Zhang A, Zhong F. Association between Homocysteine Levels and All-cause Mortality: A Dose-Response Meta-Analysis of Prospective Studies. Sci Rep. 2017 Jul 6;7(1):4769.
González S, Huerta JM, Fernández S, Patterson AM, Lasheras C. Homocysteine increases the risk of mortality in elderly individuals. Br J Nutr. 2007 Jun;97(6):1138-43.
Hoogeveen EK, Kostense PJ, Jakobs C, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes : 5-year follow-up of the Hoorn Study. Circulation. 2000 Apr 4;101(13):1506-11.
Kark JD, Selhub J, Adler B, Gofin J, Abramson JH, Friedman G, Rosenberg IH. Nonfasting plasma total homocysteine level and mortality in middle-aged and elderly men and women in Jerusalem. Ann Intern Med. 1999 Sep 7;131(5):321-30.
Petersen JF, Larsen BS, Sabbah M, Nielsen OW, Kumarathurai P, Sajadieh A. Long-term prognostic significance of homocysteine in middle-aged and elderly. Biomarkers. 2016 Sep;21(6):490-6.
Swart KM, van Schoor NM, Blom HJ, Smulders YM, Lips P. Homocysteine and the risk of nursing home admission and mortality in older persons. Eur J Clin Nutr. 2012 Feb;66(2):188-95.
Waśkiewicz A, Sygnowska E, Broda G. Homocysteine concentration and the risk of death in the adult Polish population. Kardiol Pol. 2012;70(9):897-902.
Wong YY, Almeida OP, McCaul KA, Yeap BB, Hankey GJ, Flicker L. Homocysteine, frailty, and all-cause mortality in older men: the health in men study. J Gerontol A Biol Sci Med Sci. 2013 May;68(5):590-8.
Vollset SE, Refsum H, Tverdal A, Nygård O, Nordrehaug JE, Tell GS, Ueland PM. Plasma total homocysteine and cardiovascular and noncardiovascular mortality: the Hordaland Homocysteine Study. Am J Clin Nutr. 2001 Jul;74(1):130-6.
Hi Dr. Lustgarten,
Have you had yourself tested for the MTHFR gene variant? My current understanding is that high doses of folic acid can be potentially damaging for some people and that other forms of folate are often recommended instead. Have you done any research on this yet?
Thanks for the good work,
Dax
LikeLike
Hey Dax. I have 23andme results-do you know the SNP for that? I can check…
LikeLike
I do not know the specific SNP but perhaps this link can help: https://www.snpedia.com/index.php/MTHFR.
The 23andme blog has a post downplaying MTHFR concerns (https://blog.23andme.com/health-traits/our-take-on-the-mthfr-gene/), while other sources such as Kresser (https://chriskresser.com/methylation-are-we-supplementing-too-much-with-dr-kara-fitzgerald/) and Masterjohn (https://chrismasterjohnphd.com/2017/08/12/living-with-mthfr/) feel that it’s still an issue worth considering. I haven’t personally put that much research into it yet since I don’t have any DNA tests on myself yet and it would all be purely speculative for me at this point.
If you decide to look into it further, I would be interested to hear your thoughts!
Take care,
Dax
LikeLike
Thanks Dax, I’ll get back to you about this. I need to read up on it…
LikeLike
I like the new clean blog look.
My homocysteine has been stubbornly high the two times I’ve tested (11.3 and 14.1), so I’m similarly trying to increase some of the B-vitamins.
Here’s a site that batch-reports the MTHFR stuff, I’m planning to use it once I get my 23andme back. It’s free:
https://geneticgenie.org/methylation-results
LikeLike
Let me know when you retest, Dan, I’m interested to see what works for you!
Ha, the new look is costing me $, so let’s see if it’s worth it!
LikeLike
Glad you’re back to posting on diet and health. Wish you would write a book on the subject. Maybe someday?
LikeLike
Thanks Peter, and yes on the book. Some of that is in my amazon book, but for the moment I need to spend the majority of my time on stuff that pays the bills, grants!
LikeLike
I remember you writing a post about the perils of excess methionine in the diet. If you think about the biochemistry of it, an excessive methionine intake may be related to high homocysteine (in general – perhaps not in your case). For methionine is an important methyl donor, and once that terminal methyl group is removed, it becomes homocysteine. Of course the body has a way to recycle homocysteine: folate and Vitamin B12 are needed to convert it back to methionine. So people with high homocysteine would be wise to either up their folate intake or increase their B12 intake, or both. (Taking folic acid is also fine as long as you do not have any mutations on the 677th or 1298th base pair of the gene that controls the behavior of the MTHFR enzyme. If you do – and these mutations are extremely widespread, affecting half the population! – you’ll want to steer clear of folic acid and stick with folates. Similarly, if you ever decided to supplement B12, taking it as cyanocobalamin would only be fine if you had none of the mutations mentioned above. Otherwise, methylcobalamin – or a few mussels – is the way to go.)
LikeLike
“Similarly, if you ever decided to supplement B12, taking it as cyanocobalamin would only be fine if you had none of the mutations mentioned above.”
My understanding was that the only people who should be concerned with cyano B12 were smokers (high cyanide intake already) and those who lack the gastric intrinsic factor (IF) to properly absorb cyano B12. Do you have a link to any papers describing the benefits of the methyl B12 form for MTHFR mutants?
LikeLike
I got my homocysteine level down from 12 to 6 by taking bee pollen and liver caps….b vits! The liver caps are high in b12. I can’t find clean liver so I buy the capsules that are dried liver from clean cows. I just bought your little ebook. Can’t wait to read it. Keep us posted on how you do!
LikeLike
Btw…I prefer a food based health program over supplements.
LikeLike
Very interesting, Kim. I’ll keep that in mind if TMG doesn’t work! Also, I’m not only interested in reducing homocysteine, but which approach also allows my other circulating biomarkers to stay optimal? I’m gonna find out!
LikeLike
Nice!
LikeLike
btw, if you like the book (ha, or don’t), please don’t hesitate to leave a review on Amazon!
LikeLike
So how did the (trimethyl)glycine supplementation work out?
LikeLike
Unfortunately, 4g of TMG did nothing to my homocysteine. I switched to a B6-B12-methylfolate stack, and I’m gonna retest in 2 weeks. Stay tuned, Zoltan!
LikeLike
Thanks, Michael!
LikeLike