Two blood markers of liver health are aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT). AST and ALT are proteins that are usually found inside liver cells, but when there is liver cell damage, they’re released into the blood. It’s important to note that these proteins can also be elevated in the blood because of muscle damage. The reference range for AST is 10-40 U/L, and 7-56 U/Lfor ALT, but are these values optimal for health and longevity?
In a meta-analysis that included ~9 million adults (average age, 51y) that were followed for up to 20 years, Kunutsor et al. (2014) reported the association between AST and ALT with all-cause mortality risk. For AST (4 studies, 9,046,609 subjects), 10-15 U/L was associated with maximally reduced all-cause mortality risk:

For ALT (8 studies, 9,087,436 subjects), 12-15 U/L was associated with maximally reduced all-cause mortality risk:

While these studies are relevant for middle-aged adults between ~50-70y, what about at older ages? Shown below are the AST and ALT values for adults older than 100 years (centenarians):

Interestingly, the centenarians’ AST and ALT values are not far from the meta-analysis data for middle-aged adults. For example, the centenarians’ AST values range from 17-23, and their ALT values from 9-14.
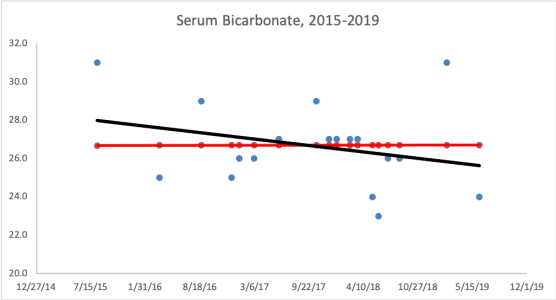
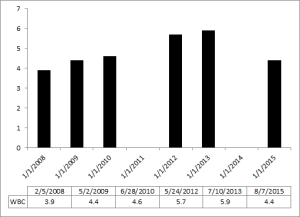
What are my my AST and ALT values? As shown below, I’ve measured them 9 times in the past 10 years. Based on the all-cause mortality and centenarian data my AST and ALT values are too high!

What am I doing to reduce my AST and ALT? Fructose is metabolized by the liver, where high amounts can increase liver cell damage, resulting in increased circulating AST and ALT (Le et al. 2009, Perez-Pozo et al. 2010). Therefore, I’ve reduced my total dietary fructose intake from ~16-18% during the 3 months prior to my last blood test (August, 2015), to ~11-14%. I plan on retesting within the next 2 months, to see if this approach works!
3/23/2016 Update: My average daily fructose intake, expressed as a percentage of total calories, for the 3-month period before my August 2015 blood test was 15.9%. During the 3-month period before my latest blood test (3/2016), my average daily fructose intake was 12.9%. Although a 3% decrease doesn’t seem like much, the difference between these 2 values is highly statistically significant (p value = 7.5E-12). Nonetheless, my liver enzymes didn’t change, with AST and ALT values of 28 and 30, respectively.
My next attempt to reduce my liver enzymes involves reducing my daily green tea intake. High doses of green tea have been shown to negatively affect the liver (Mazzanti et al. 2009). I currently drink ~6 cups of green tea per day, which may be too much. To test that hypothesis, I’ll reduce my daily green tea to 4 cups/day, and retest my liver enzymes in a few months.
12/8/2017 Update: Since 3/2016, I’ve tested my blood 7 times, and on each measurement, my ALT and AST were both still in the mid 30’s (or higher!). The green tea reduction experiment didn’t work, nor did ~30g of milk thistle seeds/day for 30+ days, nor did reducing my fructose intake to ~9% of total calories. Because I’ve tracked my nutrition in concordance with blood testing, I can look at which nutrients correlate with my liver enzymes, and reduce/increase certain foods that may impact them. Interestingly, my dietary niacin levels (x-axis), which average 41 mg/day (including all data since 2015) were strongly correlated with ALT (y-axis; r = 0.7, R^2=0.50):

Note that the RDA for niacin is ~15 mg/day for males, and my average niacin intake in more than 2-fold higher than that! This may be a case where higher than the RDA is not optimal for health. Niacin in high doses, albeit in grams, not milligrams, is well known to induce liver damage, so isn’t it possible that my 2-fold higher than the RDA niacin intake is inducing liver damage? Sometime in January, I’ll retest my liver enzymes (and everything else, of course) while reducing my dietary niacin intake from the low 40’s to the low 30’s. As I’ve mentioned in previous posts, I eat lots of mushrooms, around 300 grams at a time, which supply around 11 mg of niacin. That’s atop the list to reduce my niacin intake. Stay tuned for the data!
1/6/2018 Update: Finally, progress! On my 1/3/2018 blood test, I was able to reduce my ALT from my average 37 U/L (over 9 different tests) value to 29! To reduce it, I tried two main things: reducing my dietary niacin intake, and increasing my selenium intake.
First, as noted above, the moderately strong correlation between my dietary niacin intake with ALT suggested that reducing it may also reduce my ALT. From 12/6/2017 to 1/2/2018, I reduced my average daily niacin intake from 41 mg/day to 33.1 mg. Interestingly, in adding that data to my 9 other blood test measurements over the last 27 months, the correlation between my niacin intake with my ALT remained strong (r = 0.75, R^2 = 0.58).
Second, I also increased my dietary selenium intake, which may be involved in affecting my ALT levels. Superficially, when examining the correlation between my average selenium intake (186 mcg/day; x-axis) with ALT (y-axis), we see a very weak negative correlation (r = -0.24, R^2 = 0.06):

Then why did I increase my daily selenium intake from an average value of 186 mg/day to 207 mg/day for the 1-month period that preceded my latest blood test? I discovered that the correlation between dietary selenium density (selenium intake/100 calories) with ALT was strong (r = -0.69, R^2 = 0.47):

Why did I look at dietary selenium density instead of its absolute value? If I eat more calories, one would expect higher levels of selenium (or other nutrients), assuming I’m not eating junk. By accounting for my calorie intake, I may be better able to see how dietary nutrients affect my circulating biochemistry, rather that only looking at the absolute values for each nutrient. Also note that the correlation between niacin density (mg niacin/100 calories) was not as strong (r = 0.53, R^2 = 0.28) as the correlation between selenium density with ALT.
Is my ALT sensitive to changes in niacin, selenium, or both? Alternatively, maybe it wasn’t niacin or selenium, but an aberrant reading? I’ll keep my niacin relatively low, and my selenium relatively high, so let’s see on my next test at the end of the month.
If you’re interested, please have a look at my book!
References
Arai Y, Takayama M, Gondo Y, Inagaki H, Yamamura K, Nakazawa S, Kojima T, Ebihara Y, Shimizu K, Masui Y, Kitagawa K, Takebayashi T, Hirose N. Adipose endocrine function, insulin-like growth factor-1 axis, and exceptional survival beyond 100 years of age. J Gerontol A Biol Sci Med Sci. 2008 Nov;63(11):1209-18.
Davey A, Lele U, Elias MF, Dore GA, Siegler IC, Johnson MA, Hausman DB, Tenover JL, Poon LW; Georgia Centenarian Study. Diabetes mellitus in centenarians. J Am Geriatr Soc. 2012 Mar;60(3):468-73.
Kunutsor SK, Apekey TA, Seddoh D, Walley J. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol. 2014 Feb;43(1):187-201.
Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009 Jun;89(6):1760-5.
Lio D, Malaguarnera M, Maugeri D, Ferlito L, Bennati E, Scola L, Motta M, Caruso C. Laboratory parameters in centenarians of Italian ancestry. Exp Gerontol. 2008 Feb;43(2):119-22.
Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009 Apr;65(4):331-41.
Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). 2010 Mar;34(3):454-61.
Willcox DC, Willcox BJ, Wang NC, He Q, Rosenbaum M, Suzuki M. Life at the extreme limit: phenotypic characteristics of supercentenarians in Okinawa. J Gerontol A Biol Sci Med Sci. 2008 Nov;63(11):1201-8.
Vasto S, Scapagnini G, Rizzo C, Monastero R, Marchese A, Caruso C. Mediterranean diet and longevity in Sicily: survey in a Sicani Mountains population. Rejuvenation Res. 2012 Apr;15(2):184-8.