Here’s my latest academic publication, enjoy!



To make it easier to review the aging and all-cause mortality data for the circulating biomarkers that are contained within the biological age calculator, Phenotypic Age (see https://michaellustgarten.wordpress.com/2019/09/09/quantifying-biological-age/), here’s a checklist!
2. Creatinine: https://michaellustgarten.wordpress.com/2019/11/18/optimizing-biologic-age-creatinine/
3. Glucose: https://michaellustgarten.wordpress.com/2019/10/04/blood-glucose-whats-optimal/
4. C-reactive protein: https://michaellustgarten.wordpress.com/2019/10/19/optimizing-biological-age-crp/
5. Lymphocyte %: https://michaellustgarten.wordpress.com/2019/11/16/lympho-mortal/
6. Mean corpuscular volume (MCV): https://michaellustgarten.wordpress.com/2019/10/14/optimizing-biological-age-mcv/
7. Red cell distribution width (RDW%): https://michaellustgarten.wordpress.com/2019/09/25/optimizing-biological-age-rdw/
8. Alkaline phosphatase: https://michaellustgarten.wordpress.com/2019/10/07/alkaline-phosphatase/
9. White blood cells: https://michaellustgarten.wordpress.com/2019/10/11/blood-testing-whats-optimal-for-wbc-levels/
Creatinine is one of the 9 blood test variables included on the biological age calculator, Phenotypic Age (https://michaellustgarten.wordpress.com/2019/09/09/quantifying-biological-age/). The reference range for women and men is 0.5 – 1.1, and 0.6 – 1.2 mg/dL respectively, but within that range, what’s optimal for health and longevity?
To answer that question, it’s important to know how circulating levels of creatinine change during aging, and its association with risk of death for all causes. Creatinine increases during aging, as reported in studies of 9,389 adults (age range, 30 – 75y; Levine 2013), and in 377,686 subjects (age range, 18 – 85y; Wang et al. 2017). However, the absolute values for these changes, i.e. from 0.8 to 1.0 mg/dL, for ex., was not reported in either study.
In terms of all-cause mortality risk, creatinine levels of 0.8 mg/dL (blue line; 95% confidence interval (CI), red dotted line) were associated with the lowest risk of death for all causes, with risk being significantly reduced for creatinine values between 0.6 – 1.1 mg/dL in the 30,760 older adults (median age, 69y) of Solinger and Rothman (2013):

Note the U-shaped mortality curve for creatinine: all-cause mortality increased when it was both less than or greater than 0.8 mg/dL. More specifically, risk of death for all causes was significantly increased when serum levels of creatinine were less than ~0.55 and greater than 1.5 mg/dL.
Few studies have investigated the association between serum (or plasma) levels of creatinine with all-cause mortality risk, as most studies use creatinine in conjunction with chronological age, gender, and ethnicity to estimate kidney function (eGFR). For example, the MDRD equation (Levey et al. 2006) is commonly used to calculate eGFR, and if you’re interested in converting your creatinine levels into eGFR, here’s a link to calculate it (https://www.mdcalc.com/mdrd-gfr-equation). As creatinine goes up, eGFR goes down, and is indicative of worse kidney function. Based on that, we should expect to see an age-related decrease in kidney function, as measured by eGFR. Is this true?
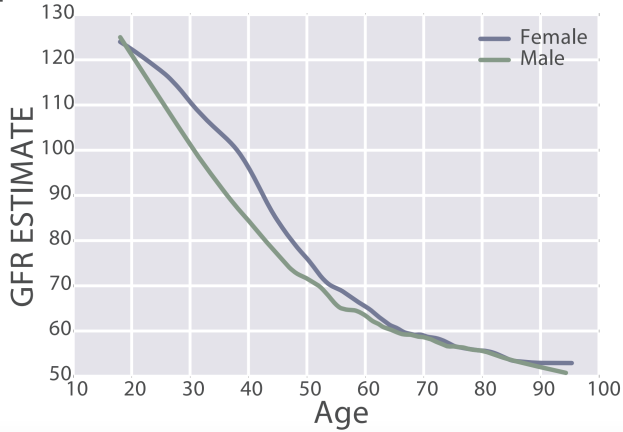
eGFR decreases during aging, from values ~125 in 20 year old women and men to ~50 mL/min/1.73m^2 in adults older than 90y in the 385,918 subjects (age range, 18 – 100y) of Wang et al. (2017):
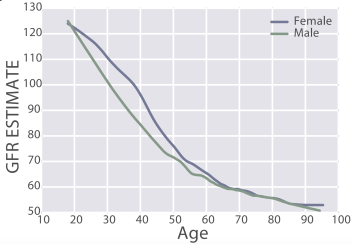
Similarly, eGFR decreased from ~90 (thick black line; 95% CI, dashed lines: 75 – 130 mL/min/1.73m^2) in young men (18-24y) to less than 70 (thick black line; 95% CI, dashed lines: 45 – 90 mL/min/1.73m^2) in men older than 75y (Baba et al. 2015):
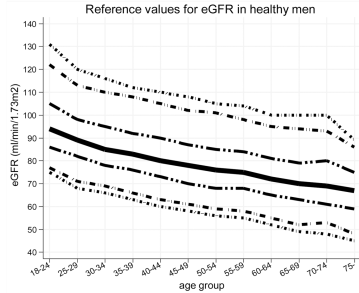
In women, eGFR decreased from values ~100 (thick black line; 95% CI, dashed lines: 70 – 135 mL/min/1.73m^2) in youth to ~70 (thick black line; 95% CI, dashed lines: 50 – 95 mL/min/1.73m^2) in women older than 75y (Baba et al. 2015):

Similar findings have been reported for the age-related decline in eGFR in other studies, including Wetzels et al. (2007). When comparing young adults (18-24 year olds) with older adults (> 85y), median eGFR values declined from ~95 to ~65 mL/min/1.73m^2 in men, and from ~90 to ~60 mL/min/1.73m^2 in women.
What’s the effect of reduced kidney function (i.e. increased creatinine, decreased eGFR) on risk of death for all causes? In a meta-analysis of 46 studies that included 2,051,158 subjects, risk of death for all causes was significantly increased when eGFR was less than 52 in women (red, below), and less than 44 in men (blue), when compared with eGFR values between 90 – 104 mL/min/1.73m^2 (95 was used as the reference; Nitsch et al. 2013):

In sum, creatinine increases during aging, which is associated with an increased all-cause mortality risk. Similarly, eGFR, which includes circulating values for creatinine, decreases during aging, and is also associated with an increased all-cause mortality risk. Therefore, if you’re tracking your creatinine levels with the goal of optimizing your biological age, it’s important to try to keep creatinine levels relatively low (i.e. around 0.8 mg/dL), and to avoid its age-related increase!
If you’re interested, please have a look at my book!
References
Baba M, Shimbo T, Horio M, Ando M, Yasuda Y, Komatsu Y, Masuda K, Matsuo S, Maruyama S. Longitudinal Study of the Decline in Renal Function in Healthy Subjects. PLoS One. 2015 Jun 10;10(6):e0129036.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247-54.
Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013 Jun;68(6):667-74. doi: 10.1093/gerona/gls233.
Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR; Chronic Kidney Disease Prognosis Consortium. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013 Jan 29;346:f324. doi: 10.1136/bmj.f324.
Solinger AB, Rothman SI. Risks of mortality associated with common laboratory tests: a novel, simple and meaningful way to set decision limits from data available in the Electronic Medical Record. Clin Chem Lab Med. 2013 Sep;51(9):1803-13. doi: 10.1515/cclm-2013-0167.
Wang Z, Li L, Glicksberg BS, Israel A, Dudley JT, Ma’ayan A. Predicting age by mining electronic medical records with deep learning characterizes differences between chronological and physiological age. J Biomed Inform. 2017 Dec;76:59-68. doi: 10.1016/j.jbi.2017.11.003.
Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age– and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007 Sep;72(5):632-7.

The percentage of lymphocytes is one of the 9 blood test variables included in the biological age calculator, Phenotypic Age (https://michaellustgarten.wordpress.com/2019/09/09/quantifying-biological-age/). The reference range for lymphocyte % is 20 – 40% of the total amount of white blood cells (WBCs), but are higher or lower values optimal for health and longevity?
To answer that question, it’s important to know how levels of lymphocytes change during aging, and its association with risk of death for all causes. In one of the earliest studies to examine how the percentage of lymphocytes changes with age, Levine (2013) reported that lymphocyte % significantly decreased during aging in 9,389 adults (age range, 30 – 75y). However, the absolute values for these changes, i.e. from 40% to 30%, for ex., was not reported.
Similarly, lymphocyte % decreased during aging in a much larger study (377,686 subjects; age range, 18 – 85y; Wang et al. 2017):
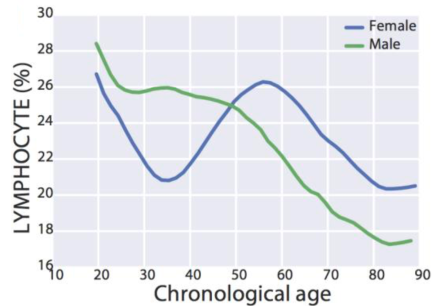
Interestingly, for women, lymphocyte % decreased from 27% to 21% from 20 – 35y, increased from 21% to 26% from 35 – 55y, then again decreased from 26% to 20% from 55y to 85y. In contrast, lymphocyte % more steadily decreased for men, from 28% to 17% from 20 – 85y.
Based on the aging data, higher values for lymphocyte % are are associated with biologic youth, whereas lower values are found in older adults. Although there are few studies that have investigated associations between lymphocyte % with aging or disease risk, in contrast, more studies have been published for absolute levels of lymphocytes.
In a small study of 106 older adults (> 85y) that were healthy (i.e. free of disease) at baseline, lymphocytes less than 1.14*10^9 cells/L (equivalent to 1140*10^6 cells/L) was associated with an increased risk of death for all causes, when compared with 1850*10^6 cells/L (Izaks et al. 2003):

In a larger study (624 subjects), lymphocytes less than 1540*10^6 cells/L was associated with a significantly shorter average lifespan (~5y; 0.5 proportion remaining below), when compared with 1540 – 2040*10^6 cells/L . Also note that survival for the group that had 1540 – 2040*10^9 lymphocytes/L was not significantly different from the group that had more than 2040*10^9 lymphocytes/L (Leng et al. 2005):
In agreement with the smaller studies, lymphocytes < 1300 and < 1200*10^6 cells/L in women and men (red and blue, far left), respectively was associated with an increased all-cause mortality risk, when compared with average lymphocyte values ~1900*10^6 cells/L (decile 5) in a larger study that included 262,394 non-smokers (age range, 37 – 73y; Welsh et al. 2018):

Collectively, these data suggest that higher values for lymphocyte % and for the absolute amount of lymphocytes may be optimal for minimizing disease risk and for maximizing longevity. If both are low, can they be raised? Circulating levels of lymphocytes are reduced during zinc deficiency (Fraker and King, 2001), so monitoring zinc intake, then increasing it to at least the RDA may be a first step towards increasing lymphocyte levels and %.
If you’re interested, please have a look at my book!
References
Fraker PJ, King LE. A distinct role for apoptosis in the changes in lymphopoiesis and myelopoiesis created by deficiencies in zinc. FASEB J. 2001 Dec;15(14):2572-8.
Izaks GJ, Remarque EJ, Becker SV, Westendorp RG. Lymphocyte count and mortality risk in older persons. The Leiden 85-Plus Study. J Am Geriatr Soc. 2003 Oct;51(10):1461-5.
Leng SX, Xue QL, Huang Y, Ferrucci L, Fried LP, Walston JD. Baseline total and specific differential white blood cell counts and 5-year all-cause mortality in community-dwelling older women. Exp Gerontol. 2005 Dec;40(12):982-7.
Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013 Jun;68(6):667-74. doi: 10.1093/gerona/gls233.
Wang Z, Li L, Glicksberg BS, Israel A, Dudley JT, Ma’ayan A. Predicting age by mining electronic medical records with deep learning characterizes differences between chronological and physiological age. J Biomed Inform. 2017 Dec;76:59-68. doi: 10.1016/j.jbi.2017.11.003.
Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, Lyall DM, Iliodromiti S, Gill JMR, Pell J, Jhund PS, Sattar N. Association of Total and Differential Leukocyte Counts With Cardiovascular Disease and Mortality in the UK Biobank. Arterioscler Thromb Vasc Biol. 2018 Jun;38(6):1415-1423. doi: 10.1161/ATVBAHA.118.310945.

My average biological age in 2019 is 12 years younger than my chronological age (46y) based on the Phenotypic Age calculator (https://michaellustgarten.wordpress.com/2019/11/01/biological-age-31-3y-chronological-age-46y/), and 16y younger based on aging.ai (https://michaellustgarten.wordpress.com/2019/11/04/years-of-biological-aging-in-the-past-4-years/). One factor that likely contributes to my relatively youthful biological age is my diet.
Shown below is my average daily dietary intake from January 1 through November 7th, 2019 (n=306 days). I weigh all of my food with a food scale, so these aren’t estimated amounts:
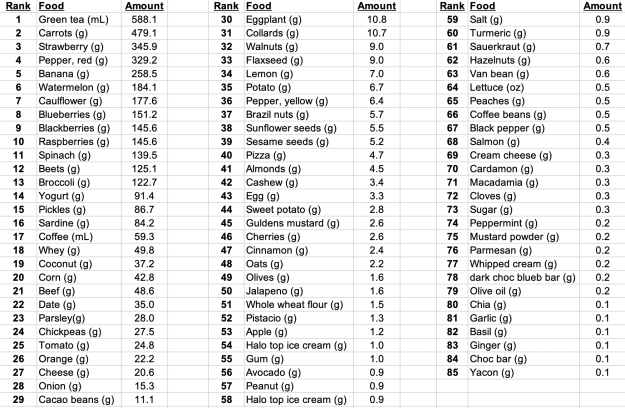
In terms of weight (or volume), green tea is atop the list, as I drink 20 oz/day. Carrots come in second place (for why, see https://michaellustgarten.wordpress.com/2018/07/06/serum-albumin-and-acm/), followed by strawberries, red bell peppers, bananas, watermelon (for the lycopene), cauliflower, blueberries, blackberries, and raspberries. Note that I mix the bananas and berries in my green smoothies, which I drink 3-4x/week, which includes spinach (#11) and parsley (#23).
What does my average daily macro- and micro-nutrient data look like for 2019?
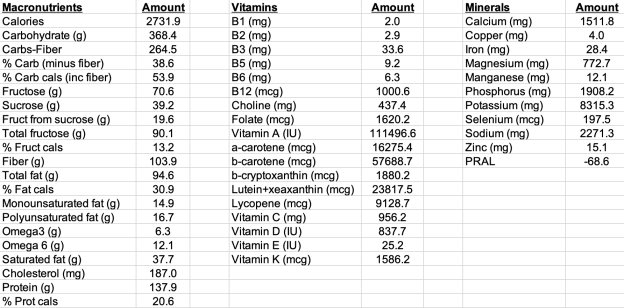
Note that I purposefully have higher than the RDA values for several nutrients, including Vitamin C (see https://michaellustgarten.wordpress.com/2019/09/19/vitamin-c-dietary-intake-and-plasma-values-whats-optimal-for-health/), Vitamin K (see https://michaellustgarten.wordpress.com/2015/05/08/eat-more-green-leafy-vegetables-reduce-mortality-risk/), selenium (see https://michaellustgarten.wordpress.com/2015/05/28/selenium-dietary-intake-and-plasma-values-whats-optimal-for-health/), and others (see michaellustgarten.com).
In terms of supplements, I use 1000 IU of vitamin D from November – May, and I take a methylfolate-methylB12-B6 supplement, to help keep my homocysteine levels low.
If you’re interested, please have a look at my book!

In an earlier post (https://michaellustgarten.wordpress.com/2018/06/26/maximizing-health-and-lifespan-is-calorie-restriction-essential/), I documented my aging.ai biologic age for 13 blood test measurements from 2016 – 2019. If you missed that post, here are those data:
 Note that note my average biologic age has slowly increased from 2016 to 2019, from 28y in 2016 (2 measurements), to 29.25y in 2017 (6 measurements), to 29.5y in 2018 (6 measurements), to 30y in my June 2019 measurement.
Note that note my average biologic age has slowly increased from 2016 to 2019, from 28y in 2016 (2 measurements), to 29.25y in 2017 (6 measurements), to 29.5y in 2018 (6 measurements), to 30y in my June 2019 measurement.
To gain more insight into my 2019 prediction for biologic age, I kept measuring. On September 17, 2019, I had my worst biological age to date, 33y, based on the blood test data below:

Seeing a biological age that high (for me) was the motivation that I needed to finally stick to a mild caloric restriction, which I hypothesized would positively affect my biological age. I wrote about this in my recent Phenotypic Age post (https://michaellustgarten.wordpress.com/2019/11/01/biological-age-31-3y-chronological-age-46y/). Did it work? Shown below is my blood test data for October 29th.

Based on that data, my biological age was 28y, and when averaging the 3 measurements in 2019 (so far!), my average biological age is 29.67y. When considering that my average biological age in 2016 was 28y, it looks like I’ve only aged ~1.7 years in 3.58 years of elapsed time!
If you’re interested, please have a look at my book!

On June 10, 2019 (for the first time) I measured all of the blood test variables that are included in the biologic age calculator, Phenotypic Age, and ended up with a biological age = 35.39y (https://michaellustgarten.wordpress.com/2019/09/09/quantifying-biological-age/).
While that value is 23% younger than my chronological age (46y), I knew that I could do better! So I tried again on September 17, 2019. Basically, the same biological age, 35.58y:

An 23% younger biological age on 2 separate dates, months apart might be good for most, but not for me. So, I tried again on October 29th, 2019, and voila, a biological age of 31.3y, which is 32% younger than my chronological age! How did I do it?

From my last blood test until my most recent blood test, I attempted a mild caloric restriction. To maintain my body weight, I require about 2800 calories per day, an amount which is based on daily body weight weighing in conjunction with daily dietary tracking. For the period of time that elapsed between my last 2 blood tests, I averaged 2657 calories/day, which is 3.2% less than the 2745 calories/day that I averaged for the dietary period that corresponded to my September blood test. That I was also in a very mild caloric restriction is confirmed by a reduction in my average body weight, which was (purposefully) down 0.7 lbs from September 17 to October 29th, when compared with the dietary period that corresponded to my September blood test (August 20 – September 17).
This is a superficial analysis of how I further reduced my biological age, but in future posts I’ll report the average dietary intake that corresponded to my relatively youthful biologic age!
If you’re interested, please have a look at my book!