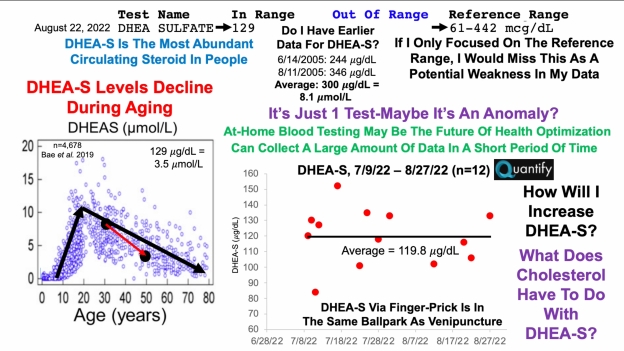
Creatinine is one of the 9 blood test variables included on the biological age calculator, Phenotypic Age (https://michaellustgarten.wordpress.com/2019/09/09/quantifying-biological-age/). The reference range for women and men is 0.5 – 1.1, and 0.6 – 1.2 mg/dL respectively, but within that range, what’s optimal for health and longevity?
To answer that question, it’s important to know how circulating levels of creatinine change during aging, and its association with risk of death for all causes. Creatinine increases during aging, as reported in studies of 9,389 adults (age range, 30 – 75y; Levine 2013), and in 377,686 subjects (age range, 18 – 85y; Wang et al. 2017). However, the absolute values for these changes, i.e. from 0.8 to 1.0 mg/dL, for ex., was not reported in either study.
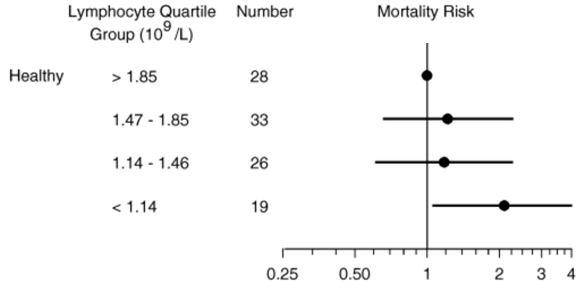
In terms of all-cause mortality risk, creatinine levels of 0.8 mg/dL (blue line; 95% confidence interval (CI), red dotted line) were associated with the lowest risk of death for all causes, with risk being significantly reduced for creatinine values between 0.6 – 1.1 mg/dL in the 30,760 older adults (median age, 69y) of Solinger and Rothman (2013):
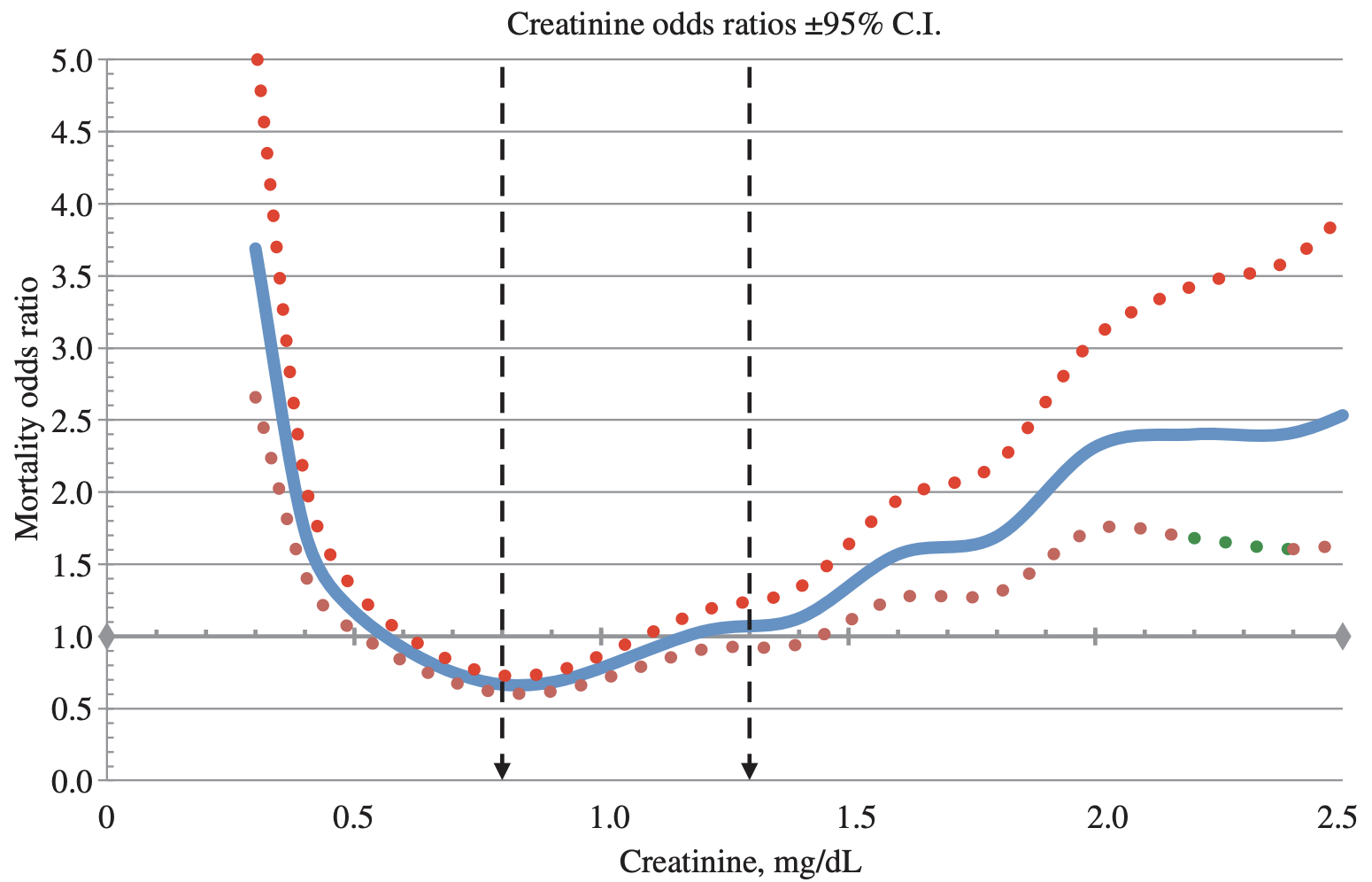
Note the U-shaped mortality curve for creatinine: all-cause mortality increased when it was both less than or greater than 0.8 mg/dL. More specifically, risk of death for all causes was significantly increased when serum levels of creatinine were less than ~0.55 and greater than 1.5 mg/dL.
Few studies have investigated the association between serum (or plasma) levels of creatinine with all-cause mortality risk, as most studies use creatinine in conjunction with chronological age, gender, and ethnicity to estimate kidney function (eGFR). For example, the MDRD equation (Levey et al. 2006) is commonly used to calculate eGFR, and if you’re interested in converting your creatinine levels into eGFR, here’s a link to calculate it (https://www.mdcalc.com/mdrd-gfr-equation). As creatinine goes up, eGFR goes down, and is indicative of worse kidney function. Based on that, we should expect to see an age-related decrease in kidney function, as measured by eGFR. Is this true?
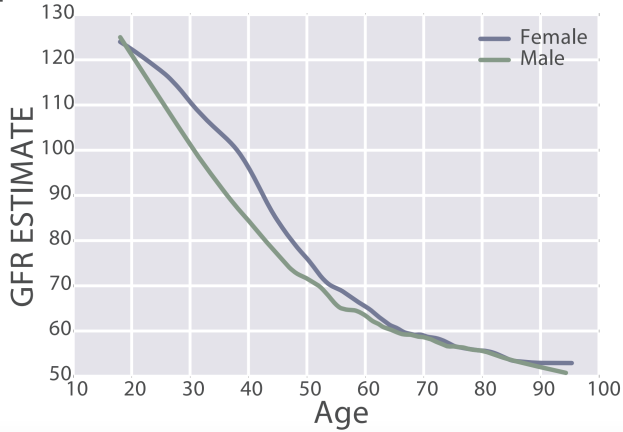
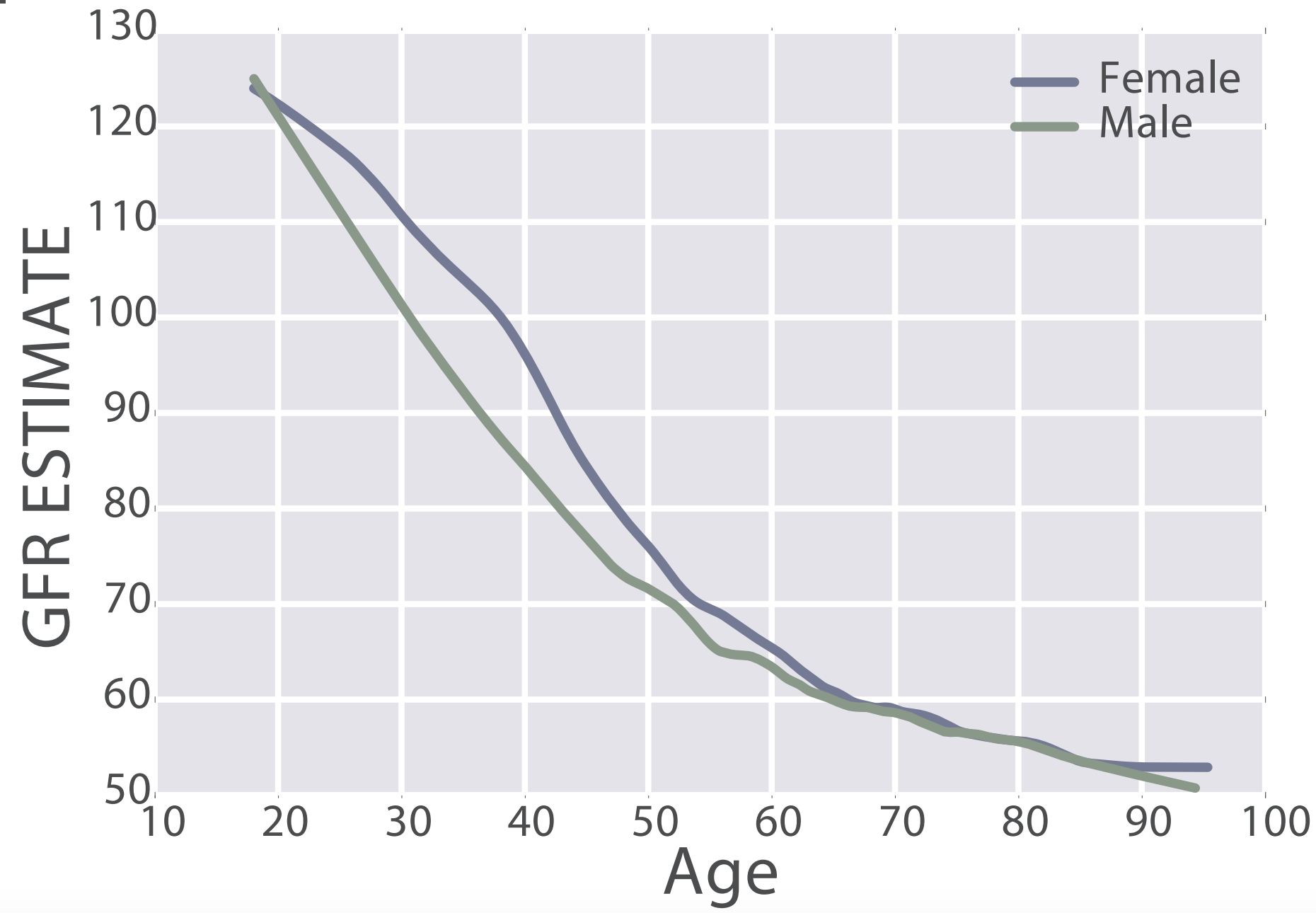
eGFR decreases during aging, from values ~125 in 20 year old women and men to ~50 mL/min/1.73m^2 in adults older than 90y in the 385,918 subjects (age range, 18 – 100y) of Wang et al. (2017):
Similarly, eGFR decreased from ~90 (thick black line; 95% CI, dashed lines: 75 – 130 mL/min/1.73m^2) in young men (18-24y) to less than 70 (thick black line; 95% CI, dashed lines: 45 – 90 mL/min/1.73m^2) in men older than 75y (Baba et al. 2015):
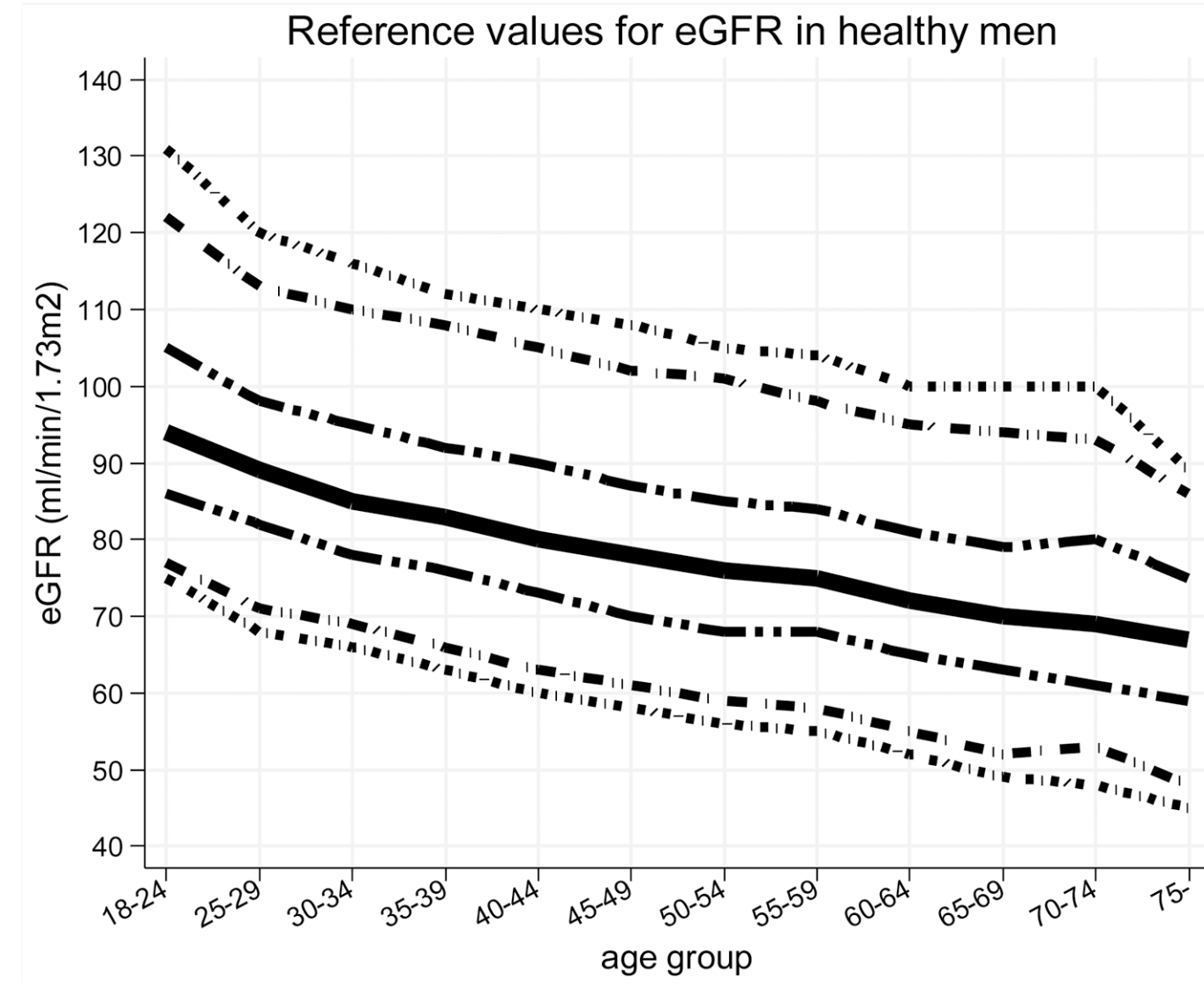
In women, eGFR decreased from values ~100 (thick black line; 95% CI, dashed lines: 70 – 135 mL/min/1.73m^2) in youth to ~70 (thick black line; 95% CI, dashed lines: 50 – 95 mL/min/1.73m^2) in women older than 75y (Baba et al. 2015):

Similar findings have been reported for the age-related decline in eGFR in other studies, including Wetzels et al. (2007). When comparing young adults (18-24 year olds) with older adults (> 85y), median eGFR values declined from ~95 to ~65 mL/min/1.73m^2 in men, and from ~90 to ~60 mL/min/1.73m^2 in women.
What’s the effect of reduced kidney function (i.e. increased creatinine, decreased eGFR) on risk of death for all causes? In a meta-analysis of 46 studies that included 2,051,158 subjects, risk of death for all causes was significantly increased when eGFR was less than 52 in women (red, below), and less than 44 in men (blue), when compared with eGFR values between 90 – 104 mL/min/1.73m^2 (95 was used as the reference; Nitsch et al. 2013):

In sum, creatinine increases during aging, which is associated with an increased all-cause mortality risk. Similarly, eGFR, which includes circulating values for creatinine, decreases during aging, and is also associated with an increased all-cause mortality risk. Therefore, if you’re tracking your creatinine levels with the goal of optimizing your biological age, it’s important to try to keep creatinine levels relatively low (i.e. around 0.8 mg/dL), and to avoid its age-related increase!
If you’re interested, please have a look at my book!
References
Baba M, Shimbo T, Horio M, Ando M, Yasuda Y, Komatsu Y, Masuda K, Matsuo S, Maruyama S. Longitudinal Study of the Decline in Renal Function in Healthy Subjects. PLoS One. 2015 Jun 10;10(6):e0129036.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247-54.
Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013 Jun;68(6):667-74. doi: 10.1093/gerona/gls233.
Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR; Chronic Kidney Disease Prognosis Consortium. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013 Jan 29;346:f324. doi: 10.1136/bmj.f324.
Solinger AB, Rothman SI. Risks of mortality associated with common laboratory tests: a novel, simple and meaningful way to set decision limits from data available in the Electronic Medical Record. Clin Chem Lab Med. 2013 Sep;51(9):1803-13. doi: 10.1515/cclm-2013-0167.
Wang Z, Li L, Glicksberg BS, Israel A, Dudley JT, Ma’ayan A. Predicting age by mining electronic medical records with deep learning characterizes differences between chronological and physiological age. J Biomed Inform. 2017 Dec;76:59-68. doi: 10.1016/j.jbi.2017.11.003.
Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age– and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007 Sep;72(5):632-7.