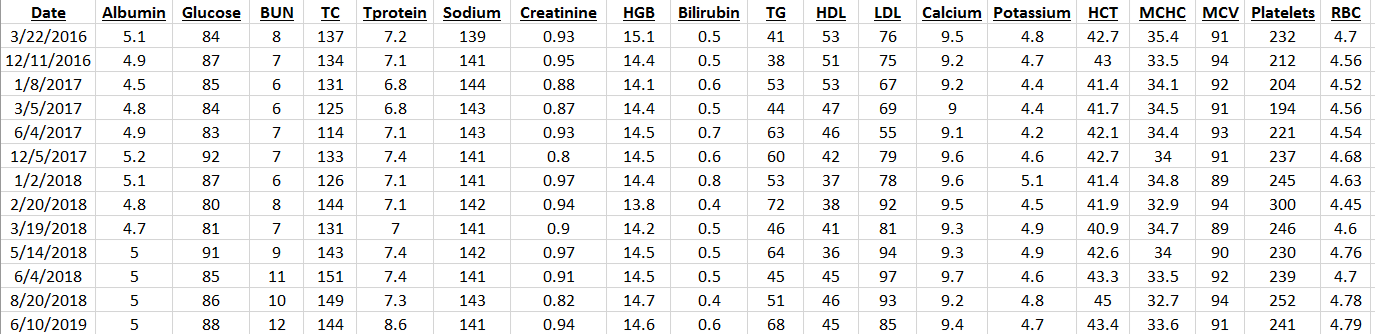
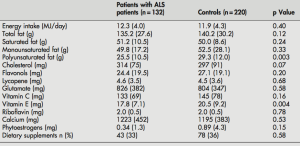
My approach towards optimal health involves yearly blood testing and tracking my results to catch changes before they become problematic. In this article, I will evaluate the published literature to propose an optimal range for circulating white blood cells (WBC).
Why is measuring WBCs important? Briefly, circulating WBCs are correlated with inflammation- inflammation increases during aging, is associated with decreased function of multiple organ systems, and is associated with an increased chronic disease risk (Cevenini et al. 2013).
As shown below, Huang et al. (2007) reported significant correlations between circulating WBCs with a marker of inflammation, C-reactive protein (CRP). This correlation was statistically significant in the whole population (14,114 subjects), in subjects older than or less than 50 years, and separately in men and women.

Based on that data, Huang et al. (2007) suggested changing the reference range (8 years ago!) for WBCs from 4-11 to 3.11-8.83 K/mm3. But within that range, what’s optimal for health and longevity? Because WBC are elevated in association with inflammation, the hypothesis would be that the lower end of the range is better, with values ~4 being optimal. Is this true?
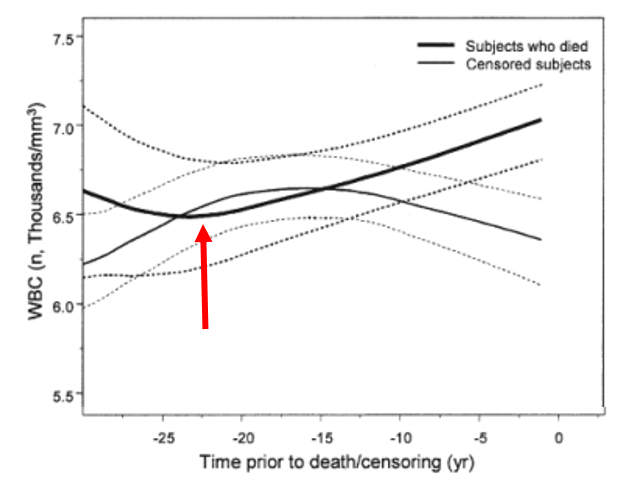
Several studies have reported that WBC values greater than 5 are associated with an increased all-cause mortality risk (Ahmadi-Abhari et al. 2013, Samet et al. 2005, Weijenberg et al. 1996). However, the best evidence for the association between WBCs with mortality risk comes from the Baltimore Longitudinal Study on Aging (BLSA), which studied 2803 men and women over a period of 44 years (Ruggiero et al. 2007). As shown below, subjects that had circulating WBC between 3.5 and 6 had decreased mortality risk, whereas below 3.5, between 6-10, and 10+ each had successively higher risk. The 0.5 point on the y-axis of the curve (survival) is defined as 50% mortality, where half of the study subjects have died. At that point, compared with subjects with WBC values between 6-10, people with values between 3.5 and 6 live ~7 years longer! So getting your WBC into that range may be a big deal for living significantly longer.

How can you reduce circulating WBCs? One way to reduce WBCs is to eat less calories, thereby reducing your body weight. As shown below, eating less calories resulted in decreased BMI and decreased WBCs in the Biosphere II project (Walford et al. 2002), almost exactly in the same pattern:

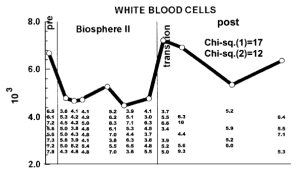
Because calorie restriction reduced WBCs from ~6.8 to 4.6, should 4.6 be considered optimal? In support of this idea, calorie restriction is well documented to increase lifespan in a variety of organisms, including flies, worms, and rodents. Although there isn’t any evidence on the long-term effects of calorie restriction (CR) on lifespan in people, it has been shown to be protective against age-related diseases, including abdominal obesity, diabetes, hypertension, and cardiovascular disease (Omodei and Fontana 2011). Therefore, a reduced WBC level may be related to the positive health-related effects of CR.
As an argument against using the CR-mediated reduction in WBC as a guide for what the optimal range should be, calorically restricted mice have decreased infection-related survival (Goldberg et al. 2015):

However, it’s important to note that infection-related survival was decreased in adult CR mice that were 40% restricted in terms of daily calories. Based on the Biosphere 2 data above, BMI was reduced from ~23 to 19, which translates into an ~18% reduction in BMI. However, whether 18% CR is better for improving infection-related survival compared with 40% CR is currently unknown.
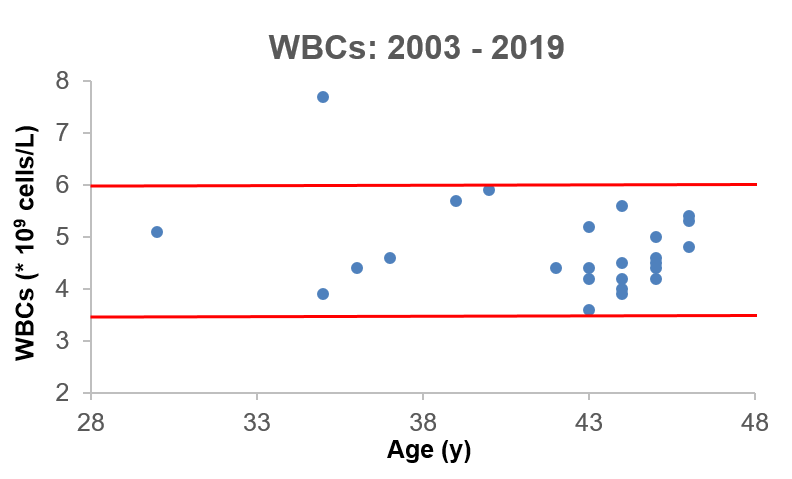
What’s my WBC level? My lowest WBC value was in 2008, at 3.9. In 4 measurements from 2008-2013 my WBC increased to 4.4, 4.6, 5.7, and 5.9. However, in my most recent blood test, they’re back down to 4.4. I have 2 possible explanations for reducing my age-related increase in WBCs. First, my body weight weight is ~10 lbs less since last year, and my 100g+ fiber diet may improve gut barrier function to keep bacteria and other stuff out of my blood that shouldn’t be there, thereby decreasing my systemic immune response.

My recent 4.4 WBC value puts me close to the CR-value (4.6), and within the optimal 3.5-6 range identified in the BLSA study. So far so good! Stay tuned for the data next year to see if my WBCs remain low or start to rise again.
If you’re interested, please have a look at my book!
References
Ahmadi-Abhari S, Luben RN, Wareham NJ, Khaw KT. Seventeen year risk of all-cause and cause-specific mortality associated with C-reactive protein, fibrinogen and leukocyte count in men and women: the EPIC-Norfolk study. Eur J Epidemiol. 2013 Jul;28(7):541-50.
Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, Di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des. 2010;16(6):609-18.
Goldberg EL, Romero-Aleshire MJ, Renkema KR, Ventevogel MS, Chew WM, Uhrlaub JL, Smithey MJ, Limesand KH, Sempowski GD, Brooks HL, Nikolich-Žugich J. Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell. 2015 Feb;14(1):130-8.
Huang ZS, Lo SC, Tsay W, Hsu KL, Chiang FT. Revision in referene ranges of peripheral total leukocyte count and differential leukocyte percentages based on a normal serum C-reactive protein level. J Formos Med Assoc. 2007 Aug;106(8):608-16.
Jee SH, Park JY, Kim HS, Lee TY, Samet JM. White blood cell count and risk for all-cause, cardiovascular, and cancer mortality in a cohort of Koreans. Am J Epidemiol. 2005 Dec 1;162(11):1062-9.
Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011 Jun 6;585(11):1537-42.
Ruggiero C, Metter EJ, Cherubini A, Maggio M, Sen R, Najjar SS, Windham GB, Ble A, Senin U, Ferrucci L. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007 May 8;49(18):1841-50.
Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002 Jun;57(6):B211-24.
Weijenberg MP, Feskens EJ, Kromhout D. White blood cell count and the risk of coronary heart disease and all-cause mortality in elderly men. Arterioscler Thromb Vasc Biol. 1996 Apr;16(4):499-503.